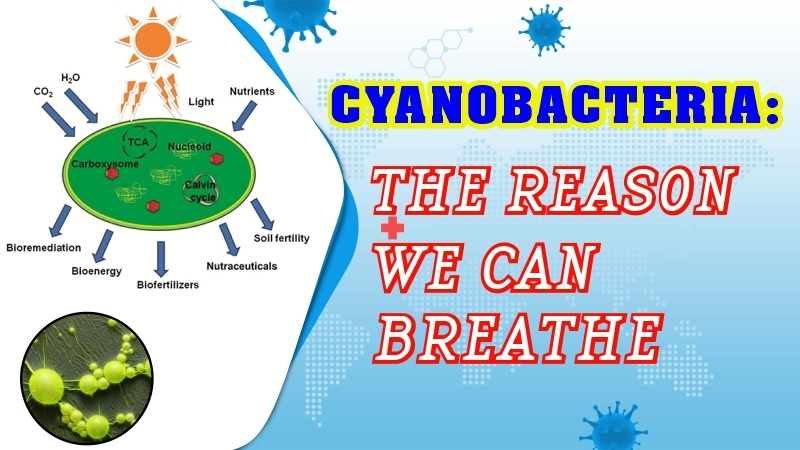
Water may boil or freeze at the same temperature. In Thermodynamics the triple point is a specific temperature and pressure at which a substance exists in equilibrium as a solid, liquid and gas at the same time. At the triple point the three phases of the substances have the same free energy and any change in temperature or pressure will cause the substance to transition from one face to another. The triple point is an important concept in the study of phase transition and is used to define the standard temperature and pressure conditions for certain substances. At this point, the three phases, liquid, ice and water vapor coexist which means that the water can boil and freeze at the same temperature.
For detail knowledge regarding this concept explore the given link: